ELECTROCHEMICAL SMOOTHING OF METALS USING ION-CONDUCTING SOLIDS IN WEAKLY CONDUCTIVE SOLUTIONS
Abstract
For applications in dental technology, it is very important to achieve a homogeneous, smooth surface without affecting the delicate components, e.g. side clasps of dental parts. Conventional polishing baths for smoothing cobalt-chrome dental parts can smooth the surface, but the result can be very uneven, de-pending on the shape of the workpiece. As an alternative, weakly conducting electrolytes with ion-exchanging solid particles based on styrene copolymers and sulfonated divinylbenzene have been available on the market for several years. This article draws a direct comparison between both methods, in terms of the electropolishing result and the surface quality achieved, using optical microscopy and roughness measurements.
It compares a polishing bath containing sulphuric acid and ethylene glycol (Megalyt, developed by Mega-dental) with an electrolyte based on ion-exchanging polymers (MFB Grey/DL4, developed by OTEC Präzi-sionsfinish GmbH).
Introduction
Electropolishing is a technical process in which the roughness of a metal surface is reduced by means of targeted electrochemical dissolution using an anodic current in an electrolyte. Unlike mechanical po-lishing, electropolishing does not need any abrasives, which would change the crystalline structure of the workpiece and become engrained in the surface. It is therefore possible to smooth delicate and complex-shaped workpieces without impurities getting in and without mechanical deformation. These advantages have led to increasing commercial use of electropolishing in recent years, particularly for medical implants and in dentistry.
Although the principle of smoothing a surface by means of anodic dissolution was already described at the beginning of the 20th century by Beutel [1] and Spitalski [2], most of the electropolishing solutions that are still used today are based on the studies carried out by Figour [3] and Jacquet [4] in the 1930s. Systematic investigations during the following decades show that electropolishing can be attributed to the formation of a diffusion-limited layer, which causes the removal of micro-roughness, in particular [5 – 12]. According to Landolt [13], Yang [14] and Han [15], the formation of this layer can be explained by the following models:
- Salt film mechanism: Formation of a saturated metallic salt film or a passive layer. The metal dissolution reaction speed is limited by the diffusion of the metallic ions through this film into the electrolytes.
- Acceptor mechanism: Reaction of the metal (M) with an acceptor (A) to form a complex MxAy. The metal dissolution reaction speed is limited by the diffusion of the acceptor to the metal surface.
To get the best possible smooth and shiny surface, the electropolishing process, the electrolyte and the workpiece material must be well matched [14, 15]. There are numerous electrolyte systems that are ma-de up of mixtures of strong acids, neutral solutions or non-aqueous solutions [16]. These are generally characterised by the fact that the liquid electrolyte solution dissolves the workpiece by applying anodic potential, thereby removing material and smoothing the surface. This is why these are referred to as po-lishing baths. The disadvantages of these polishing baths, however, are that they are often very corrosive and contain harmful components, and that they tend to produce a lot of gas at the electrodes. In additi-on, shielding of the electric field means that inner areas of complex-shaped components are processed less effectively or are not processed at all.
Ion-exchanging polymers based on styrene copolymers and sulfonated divinylbenzene, which allow much better and more homogeneous processing of the surface, are an alternative to this [17, 18]. Metal is re-moved when a connection that is conductive via the polymer particles is created between the workpiece (anode) and a cathode. These particles can thus be used dry [18, 19] or suspended in a non-conductive liquid [20]. This means that more environmentally friendly and less corrosive chemicals can be used than with conventional electropolishing in polishing baths. However, since a conductive connection is formed randomly and chaotically, material abrasion is slower than with the polishing bath. Local pitting can also occur as a result of particles sticking to the surface [17].
A process, which has been commercially available for about 4 years, in which ion-conducting polymers in the form of ion exchangers are mixed in a solution with conductive, surfactant components, offers a compromise between both methods. The electrolytic contact is produced not only by the polymer partic-les, but also by the electrically conductive solution. This means that a higher level of abrasion can be achieved in the same process time. One example of this is the mixture MFB Grey/DL4, developed by O-TEC Präzisionsfinish GmbH, which consists of the ion-conductive solid particles mentioned above (co-polymer of styrene and sulfonated divinylbenzene, MFB Grey) [21] and a mixture of ethylene glycol, petro-leum, isoparaffins, surfactants, wetting agents and glycerine (DL4) [22]. Although DL4 has a certain conductivity, it does not contribute directly to material abrasion, but only ensures better electrical contact between the particles (Fig. 1).
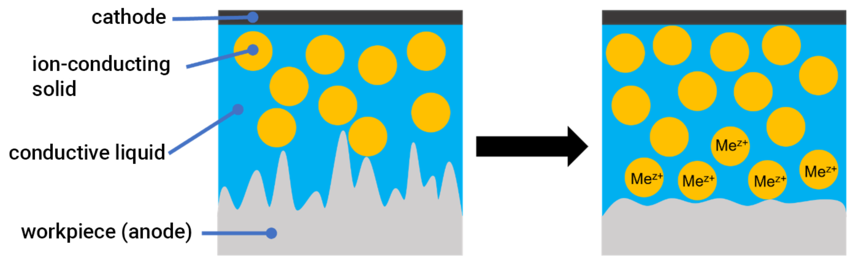
Figure 1. Schematic drawing showing the dissolution of a metal surface in MFB Grey/DL4 electrolytes. Metallic ions (Mez+) are absorbed by the ion-conducting solid during the electropolishing process. The conductive liquid reduces the resistance of the electrolyte between the anode, ion-conducting solids and the cathode.
This article draws a direct comparison between surfaces of dental castings that were treated using con-ventional electropolishing in the polishing bath (Megalyt, developed by Megadental [23]) and with floating ion-conductive polymers (MFB Grey/DL4, developed by OTEC Präzisionsfinish GmbH).
RESULTS
Figure 2 shows a dental part made of a CoCr alloy before electropolishing. To allow a better compari-son, the surfaces were sand-blasted beforehand to create a homogeneous, comparable surface. The challenge when it comes to electropolishing this workpiece is to smooth the large areas on the front (Fig. 2 a and b) and back (Fig. 2 c and d) of the workpiece uniformly without rounding the side clasps (Fig. 2 a and c) too much so that the dimensional accuracy of the workpiece is retained.bt.
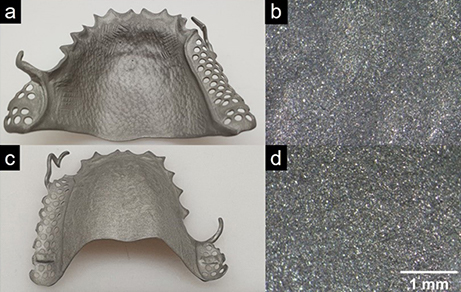
Figure 2. Photographs (a and c) and microscopic images (15x magnification) (b and d) of the front (a and b) and back (c and d) of a dental part before electropolishing.
Figure 3 shows the dental part following electropolishing (20 min, 20 V, 6 A) using conventional electro-lyte. It is clearly evident that although the side clasps and the back (Fig. 3 c and d) were smoothed very well and look shiny, the inner palate surface on the front (Fig. 3 a and b) still looks matte and rough des-pite becoming lighter in appearance. Based on the average roughness values Ra listed in Table 1, we can confirm the following: While the Ra value of the back is significantly reduced compared to the unproces-sed workpiece, the value of the front remains almost unchanged.
One explanation for this is that material abrasion is much slower in these inner areas due to electrolyte resistance, which means that these areas are not processed or are not processed well in the process time considered here. This could be corrected by introducing another auxiliary cathode, but this is tech-nically much more complex.
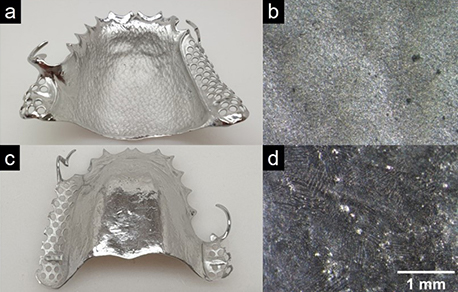
Figure 3. Photographs (a and c) and microscopic images (15x magnification) (b and d) of the front (a and b) and back (c and d) of a dental part after electropolishing for 20 minutes at 20 V in Megalyt (Megadental).
Figure 4 shows the dental part following electropolishing with ion-exchanging polymer particles (20 min, 20 V, 4 A).
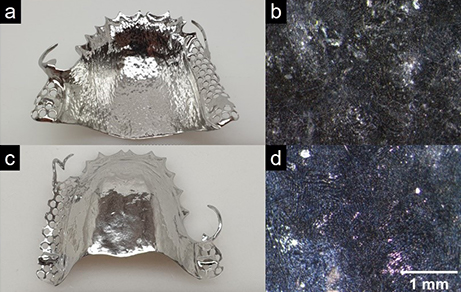
Figure 4. Photographs (a and c) and microscopic images (magnification: 15 x) (b and d) of the front (a and b) and back (c and d) of a dental part after electropolishing for 20 minutes at 4 A in MFB Grey/DL4 electrolyte (OTEC). The reflective surface makes the microscopic images in b and c very dark.
Both the side clasps and the back as well as the inner palate surface were smoothed effectively using the process and look shiny (Fig. 4 a and c). The average roughness values (Table 1) show not only a lower Ra value on the front, but also a significant reduction in the roughness value on the back in the same processing time.
Although material abrasion only occurs when the roughness peaks come into contact with the solids in MFB Grey (Fig. 1), the better electrical contact between the components as a result of the interaction of the ion-conducting solids and the supporting solution means that both contours near the cathode and areas further away, e.g. inner surfaces, are smoothed uniformly.
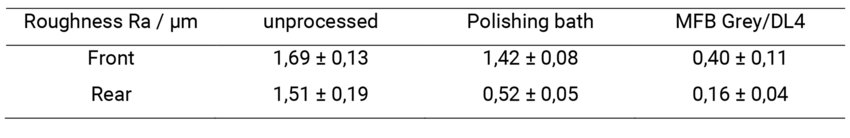
Table 1. Average roughness Ra of the front and back of a dental part before electropolishing, after electropolishing in the po-lishing bath (20 min, 20 V) and in MFB Grey/DL4 electrolyte (20 min, 20 V, 4 A).
SUMMARY
In this article, dental parts were used to demonstrate that the use of ion-conducting solids as an electrochemically active medium in an electrically conductive solution allows better and more homo-geneous smoothing than a conventional acidic polishing bath in the same processing time. The more homogeneous surface processing using MFB Grey/DL4 (OTEC Präzisionsfinish GmbH) can be explained by the fact that material abrasion is not caused by the solution itself, but primarily by the solid particles when they come into contact with the roughness peaks. Uniform smoothing in the polishing bath can be achieved by introducing another auxiliary cathode, but this is technically more challenging than changing the electropolishing medium.
In addition to the qualitative improvement of the surface quality, MFB Grey/DL4 does not contain any free acids, which means that it is less corrosive and more environmentally friendly than a comparable acid-based electrolyte. Furthermore, the use of this electrolyte is not only limited to CoCr alloys, but has already been tested successfully on copper alloys, brass and silver.
References
[1] E. Beutel in Publications of the Chem. Tech. Department of the Kais. Königliches Lehrmittelbüro, Vienna, 1907.
[2] Spitalsky, German Patent No. 225.873, 1910.
[3] H. Figour, P. A. Jacquet, French Patent No. 707526, 1930.
[4] P. A. Jacquet, Nature 1935, 135, 1076.
[5] J. Edwards, J. Electrochem. Soc. 1953, 100, 189C.
[6] W. C. Elmore, J. Appl. Phys. 1939, 10, 724.
[7] C. L. Faust, J. Electrochem. Soc. 1949, 95, 62C‐72C.
[8] D. R. Gabe, Metallography 1972, 5, 415.
[9] K. B. Hensel, Met. Finish. 2002, 100, 425.
[10] P. A. Jacquet, Metall. Rev. 1956, 1, 157.
[11] C. Wagner, J. Electrochem. Soc. 1954, 101, 225.
[12] J. Toušek, Corros. Sci. 1975, 15, 113.
[13] D. Landolt, Electrochim. Acta 1987, 32, 1.
[14] G. Yang, B. Wang, K. Tawfiq, H. Wei, S. Zhou, G. Chen, Surf. Eng. 2017, 33, 149.
[15] W. Han, F. Fang, Int. J. Mach. Tools Manuf. 2019, 139, 1-23.
[16] M. Buhlert, Electropolishing - Electrolytic brightening, smoothing and deburring of stainless steel, steel, brass, copper, aluminium and titanium, 2nd. Edition, Leuze Verlag, 2017.
[17] N.K. Krioni, A.D. Mingazhev, V.A. Gafarova, J. Phys.: Conf. Ser. 2021, 1891, 012028.
[18] P. Sarsanedas Millet, Patent No. WO2017186992A1, 2017.
[19] Y. Cheng, L. Wang, S. Yu, R. Min, J. Alloys Compd. 2021, 854, 157269.
[20] M. Sarsanedas Gimpera, G. Riu Perdrix, J. J. Roa Rovira, Patent No. ES2904576A1.
[21] OTEC Präzisionsfinish GmbH, MFB 0.5 amber, MFB 0.5 grey, MFB 1.0 grey, Straubenhardt, 2022 (revised on: 16/05/2022). – Safety data sheet
[22] OTEC Präzisionsfinish GmbH, DL 4, Straubenhardt, 2022 (revised on: 14/01/2022). – Safety data sheet
[23] megadental GmbH, megalyt polishing electrolyte, Büdingen, 2021 (version: 21/09/2021). – Safety data sheet




